Translocation intermediates of ubiquitin through an α-hemolysin nanopore: implications for detection of post-translational modifications†
Abstract
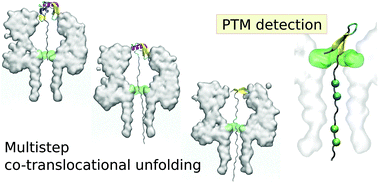
Nanopore based sensors constitute a promising approach to single molecule protein characterization being able, in principle, to detect sequences, structural elements and folding states of proteins and polypeptide chains. In narrow nanopores, one of the open issues concerns the coupling between unfolding and translocation. Here, we studied the ubiquitin translocation in an α-hemolysin nanopore, the most widely used pore for nanopore sensing, via all-atom molecular dynamics simulations. We completely characterize the co-translocational unfolding pathway finding that robust translocation intermediates are associated with the rearrangement of secondary structural elements, as also confirmed by coarse grained simulations. An interesting recurrent pattern is the clogging of the α-hemolysin constriction by an N-terminal β-hairpin. This region of ubiquitin is the target of several post-translational modifications. We propose a strategy to detect post-translational modifications at the N-terminal using the α-hemolysin nanopore based on the comparison of the co-translocational unfolding signals associated with modified and unmodified proteins.


 Please wait while we load your content...
Please wait while we load your content...