Membrane potential drives direct translocation of cell-penetrating peptides†
Abstract
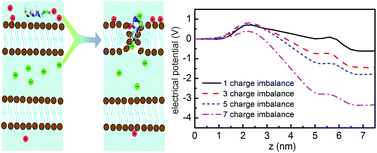
Cell-penetrating peptides (CPPs) are frequently employed as drug delivery agents with rapid cellular uptake, however, the uptake mechanism and the detailed translocation pathway are at present not completely understood. Both endocytosis and direct translocation through membrane pores have been observed in experiments and simulations under different conditions. Here we report the molecular dynamics simulations providing evidence for the direct translocation of CPPs across the membrane driven by the membrane electrostatic potential. The local membrane potential can be produced by the ion concentration imbalance across the membrane, which is ubiquitous in biological environments. Moreover, if positively charged CPPs are adsorbed on the membrane, this further enhances the membrane potential, opening membrane pores through which CPPs can be instantly transported in a chain-like configuration. The classical nucleation theory is applied to estimate the translocation time by calculating the changes in the free energy upon transferring CPPs across the membrane at different potentials, showing good agreement with available experimental measurements. The revealed CPP translocation mechanism can be broadly relevant for cellular processes in biology.


 Please wait while we load your content...
Please wait while we load your content...