Self-assembly of 5,6-dihydroxyindole-2-carboxylic acid: polymorphism of a eumelanin building block on Au(111)†
Abstract
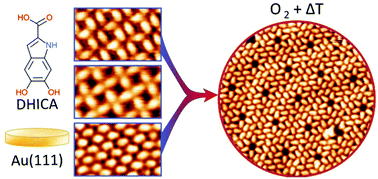
Investigating two-dimensional (2D) self-assembled structures of biological monomers governed by intermolecular interactions is a prerequisite to understand the self-assembly of more complex biomolecular systems. 5,6-Dihydroxyindole carboxylic acid (DHICA) is one of the building blocks of eumelanin – an irregular heteropolymer and the most common form of melanin which has potential applications in organic electronics and bioelectronics. By means of scanning tunneling microscopy, density functional theory and Monte Carlo calculations, we investigate DHICA molecular configurations and interactions underlying the multiple 2D patterns formed on Au(111). While DHICA self-assembled molecular networks (SAMNs) are dominated by the hydrogen bonding of carboxylic acid dimers, a variety of 2D architectures are formed due to the multiple weak interactions of the catechol group. The hydroxyl group also allows for redox reactions, caused by oxidation via O2 exposure, resulting in molecular rearrangement. The susceptibility of the molecules to oxidation is affected by their SAMNs architectures, giving insights on the reactivity of indoles as well as highlighting non-covalent assembly as an approach to guide selective oxidation reactions.


 Please wait while we load your content...
Please wait while we load your content...