Structure–activity relationship of nanostructured ceria for the catalytic generation of hydroxyl radicals†
Abstract
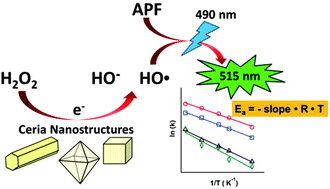
Reactive oxygen species (ROS) are powerful oxidants generated in both biological systems and natural environments. Though enzyme-mimic activity and Fenton-like reactions have been postulated to explain how ceria nanoparticles and ROS are involved in the catalytic decomposition of hydrogen peroxide (H2O2), the corresponding reaction kinetics for this reaction have not yet been completely resolved. Here we present our investigation of the structure–activity relationship of ceria nanostructures for the generation of hydroxyl radicals through the catalytic decomposition of H2O2. Different nanostructured ceria including nanorods (NR), nanocubes (NC), and nanooctahedra (NO), together with commercial ceria, were examined to elucidate the relationship between the morphology and reaction kinetics. The initial relative production rates of hydroxyl radicals over different ceria nanostructures were determined using fluorescence measurements and were applied to obtain the apparent activation energy for their intrinsic activity comparisons. The activity trend of the order: ceria NR > ceria NC > ceria NO > commercial ceria was observed. This trend was rationalized and assessed using activity descriptive factors including the intensity ratio of Raman bands of vibration modes due to atomic defects, the percentage of surface Ce3+ content, and the average coordination number of oxygen anions surrounding each cerium cation in the ceria samples.


 Please wait while we load your content...
Please wait while we load your content...