Influence of surface chemistry on optical, chemical and electronic properties of blue luminescent carbon dots†
Abstract
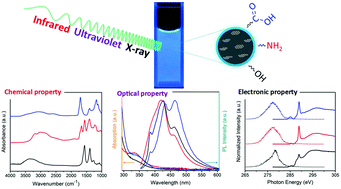
Carbon dots have attracted much attention due to their unique optical, chemical and electronic properties enabling a wide range of applications. The properties of carbon dots can be effectively adjusted through modifying their chemical composition. However, a major challenge remains in understanding the core and surface contributions to optical and electronic transitions. Here, three blue luminescent carbon dots with carboxyl, amino and hydroxyl groups were comprehensively characterized by UV-vis absorption and emission spectroscopy, synchrotron-based X-ray spectroscopy, and infrared spectroscopy. The influence of the surface functionality on their fluorescence was probed by pH-dependent photoluminescence measurements. Moreover, the hydrogen bonding interactions between water and the surface groups of carbon dots were characterized by infrared spectroscopy. Our results show that both core and surface electronic states of blue luminescent carbon dots contribute to electronic acceptor levels while the chemical nature of the surface groups determines the hydrogen bonding behavior of the carbon dots. This comprehensive spectroscopic study demonstrates that the surface chemistry has a profound influence on the electronic configuration and surface–water interaction of carbon dots, thus affecting their photoluminescence properties.


 Please wait while we load your content...
Please wait while we load your content...