Efficient base-free direct oxidation of glucose to gluconic acid over TiO2-supported gold clusters†
Abstract
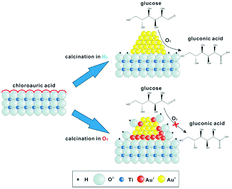
The transformation of renewable natural resources is an appealing and sustainable protocol to minimize fossil fuel consumption. Here, a simple incipient wetness protocol is developed to prepare ultrasmall gold clusters, immobilized on TiO2 (particle size: 1.2–1.7 nm), using anthranilic acid as a stabilizing agent. The Au clusters can be reduced to metallic Au0 (Au/TH-150 and Au/TH-200) during 150 and 200 °C annealing in the presence of H2 gas, while the Au clusters are converted to Auδ+ species in air at 200 and 500 °C (Au/TA-200 and Au/TA-500), a conclusion supported by XPS and low-temperature (−150 °C) Operando-DRIFTS analysis. Au/TA-200 and Au/TA-500 showed inactivity in the base-free direct oxidation of glucose. For comparison, Au/TH-150 and Au/TH-200 exhibited salient catalytic performance (87–92% conversion and 95–97% selectivity for gluconic acid), revealing that glucose oxidation occurs preferentially on the Au0 species. The turnover frequency (TOF) of Au/TH-150 reaches 1908 molreacted glucose molAu−1 h−1, which is much higher than that of commercial Pd–Bi/C under alkaline conditions (TOF: 1298 molreacted glucose molPd−1 h−1, pH 9.5). The apparent activation energies are 37 (over Au/TH-150) and 47 kJ mol−1 (Au/TH-200), comparable to the unsupported Au colloids, indicating that the oxidation should occur at the Au surface rather than at the perimeter interface between the Au clusters and the supports.


 Please wait while we load your content...
Please wait while we load your content...