Biosynthesis and chemical diversity of β-lactone natural products
Abstract
Covering: up to 2018
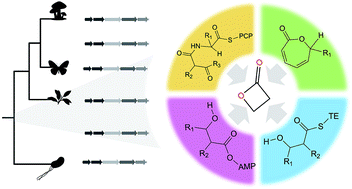
β-Lactones are strained rings that are useful organic synthons and pharmaceutical warheads. Over 30 core scaffolds of β-lactone natural products have been described to date, many with potent bioactivity against bacteria, fungi, or human cancer cell lines. β-Lactone natural products are chemically diverse and have high clinical potential, but production of derivatized drug leads has been largely restricted to chemical synthesis partly due to gaps in biochemical knowledge about β-lactone biosynthesis. Here we review recent discoveries in enzymatic β-lactone ring closure via ATP-dependent synthetases, intramolecular cyclization from seven-membered rings, and thioesterase-mediated cyclization during release from nonribosomal peptide synthetase assembly lines. We also comprehensively cover the diversity and taxonomy of source organisms for β-lactone natural products including their isolation from bacteria, fungi, plants, insects, and marine sponges. This work identifies computational and experimental bottlenecks and highlights future directions for genome-based discovery of biosynthetic gene clusters that may produce novel compounds with β-lactone rings.


 Please wait while we load your content...
Please wait while we load your content...