Water and oxoanion encapsulation chemistry in a 1H-pyrazole azacryptand†
Abstract
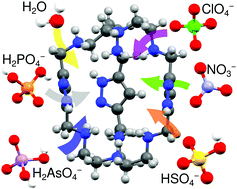
Anion complexes of the cryptand built with the tripodal amine tris(2-aminoethyl)amine, known as tren, with water and several oxoanions of biological and environmental interest (nitrate, sulfate, phosphate, perchlorate and arsenate) have been crystallized from aqueous solution and resolved with single-crystal X-ray diffraction. All crystals show guest species encapsulated in the interior of the cavity as well as, in some cases, sitting in the grooves defined by the arms of the macrocycle. Hydrogen bonding and electrostatic interactions play a major role in anion binding to the host. The macrocycle is able to encapsulate anions in a wide range of protonation degrees. Solution studies have been carried out to show that those interactions happen also in solution in addition to the solid state. The results show that the receptor binds consistently to the ligand in a wide range protonation degrees.


 Please wait while we load your content...
Please wait while we load your content...