One-pot synthesis of benzotripyrrole derivatives from 1H-pyrroles†
Abstract
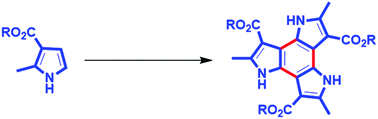
Herein, we report, for the first time, the synthesis of C3h symmetric benzotripyrroles having unprotected pyrrolic nitrogens in a single step from a known starting material, i.e. 2-methyl-1H-pyrrole-3-carboxylates, and characterized the isolated intermediate compounds to elucidate the mechanism. Further, computational studies were performed to probe the dual descriptor and aromaticity to understand the reactivity of the pyrrole derivatives towards cyclization and the stability of the benzotripyrrole.


 Please wait while we load your content...
Please wait while we load your content...