“Naked-eye” detection of CN− from aqueous phase and other extracellular matrices: an experimental and theoretical approach mimicking the logic gate concept†
Abstract
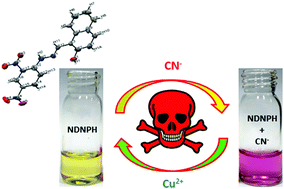
Herein, a naphthaldehyde-hydrazine-based colorimetric chemosensor (NDNPH) ((E)-1-((2-(2,4-dinitrophenyl)hydrazono)methyl)naphthalen-2-ol) was designed, synthesised and evaluated for target-specific anion detection through the H-bonding mechanism. The chemosensor exhibited high selectivity towards lethal anions, such as CN−, with a 1 : 1 binding stoichiometry and a 1.2 × 107 M−1 binding constant. The NDNPH sensor showed high selectivity towards CN− with a distinct naked eye colour change from light yellow to purple. The detection limit in the aqueous phase was calculated to be as low as 1.26 μM, which was much lower than the permissible limit. The NDNPH sensor was equally responsive in different biological matrices such as fresh bovine serum and human blood plasma. This shows the bioapplicability of the chemosensor, which is of immense importance in analytical domains in the modern era. In addition to several spectroscopic studies, the DFT studies together with the electrostatic potential surface analysis and Loewdin spin population calculation confirmed the binding behaviour of the sensor molecule with the targeted analyte. Furthermore, the reversible UV-vis responses of the synthesized chemosensor NDNPH towards CN− and Cu2+ could mimic several molecular logic functions and hence were used to fabricate several complex electronic circuitries based on Boolean Algebra. Moreover, “test strips” based on the NDNPH sensor were fabricated, which might act as a conventional and efficient cyanide detection kit for real-field detection without the need for additional instruments by virtue of the “Dip-Stick” approach. In summary, this simple, cost-effective, azomethine-based simple Schiff base molecule detects biologically lethal anions (CN−) in various biological matrices with real-field application in analytical and engineering sciences.


 Please wait while we load your content...
Please wait while we load your content...