A new recipe for the rapid microwave synthesis of high quantum yield Mn2+-doped ZnGa2O4 phosphors for potential forensic applications†
Abstract
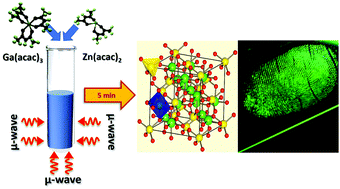
A new microwave technique was used to synthesize MnxZn(1−x)Ga2O4 nanoparticles within 5 minutes through a pH independent, environment friendly method employing metal–organic precursors. At temperatures as low as 175 °C, the as-prepared sample was phase pure and crystalline with crystallite sizes of ∼5–7 nm. The Mn2+ doping concentration was optimized and the sample that showed the highest photoluminescence (PL) intensity was used to investigate thermal effects on the structural and optical properties of the material. Rietveld refinement was performed on the sample annealed at 1200 °C and the global cationic inversion was determined to be ∼1.9%. Crystallite sizes increased from 5 to 44 nm with increasing annealing temperature while the bandgap blue-shifted from ∼3.9 eV to ∼4.7 eV. The improvement in material crystallinity resulted in a marked increase in the emission intensities, suggesting that Mn2+ emissions were crystalline environment dependent. The site symmetry and local field environment of the Mn2+ ions were investigated from the electron paramagnetic resonance hyperfine structure while the PL peak obtained at 503 nm was ascribed to the 4T1 (G) → 6A1 (S) spin-forbidden transitions in Mn2+. It was shown that cationic inversion of Mn2+ affected its PL emission that manifested as an asymmetry in the emission profile. An internal quantum yield of 64%, obtained for the sample annealed at 1200 °C, is one of the highest reported values for a powder ceramic and its potential use as a phosphor in latent fingerprint detection was demonstrated. X-ray photoelectron spectroscopy revealed a 30% inversion in Zn2+ occupancy, suggesting that microwave synthesis has a role to play in determining the site occupancy of the cations. This work demonstrates a technologically important, green, and swift technique for developing phosphors for application in forensics, displays, imaging, etc.


 Please wait while we load your content...
Please wait while we load your content...