Synthesis of energetic salts containing three heterocyclic anions by a one-pot condensation reaction†
Abstract
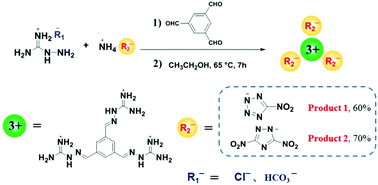
Heterocyclic energetic salts are extensively used in energetic materials due to their excellent molecular designability. Generally, most inorganic or organic anionic energetic ionic salts contain only one anion or two anions at most. Thus far, energetic ion salts containing three organic energetic anions have not been reported. In this study, we develop a new method for the preparation of energetic ion salts containing three heterocyclic anions via a one-step one-pot condensation reaction. The proposed strategy is based on aldehydes, aminoguanidine salts, and heterocyclic energetic ionic salts. In this work, energetic salt 1 containing three 5-nitrotetrazole anions and energetic salt 2 containing three 3,5-dinitro-1,2,4-triazole anions were successfully synthesized. The results of NMR, IR, and MS proved that the products (1 and 2) contain three heterocyclic anions. The energetic test results indicate that products 1 and 2 have extremely high decomposition temperatures (250 °C and 308 °C) and exhibit low sensitivities (for both 1 and 2, FS > 360 N, IS > 40 J). Meanwhile, their detonation velocities (7306 m s−1 and 7375 m s−1) and detonation pressures (18.3 GPa and 19.7 GPa) are better than those of TNT. This indicates that 1 and 2 have great potential to be applied as heat-resistant insensitive explosives. In addition, the X-ray diffraction results of the non-energetic, p-toluenesulfonic acid anions of product 3 not only further verify the structure of the three heterocyclic anions mentioned above, but also confirm the universality of this method.


 Please wait while we load your content...
Please wait while we load your content...