Internal pressure and state assessment of the inherent macroscopic force fields of liquids
Abstract
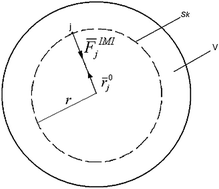
A theoretical approach is proposed for describing the nature of the internal pressure of liquids (Pi), which allows us to overcome the uncertainty of its definition and propose a new thermodynamic scale for liquids. It is shown that internal pressure is created by the molecular macroscopic force field of a liquid-phase system and is equal to a force applied from the field side to an arbitrary unit surface within the body of the liquid that is orthogonal to the surface force field vectors. The relationship between internal pressure and cohesive energy density is discussed. In this regard, concerning the physical sense, internal pressure is a force parameter, while cohesive energy density is an energy parameter. A hypothesis is proposed that the value of the temperature coefficient of the internal pressure (∂Pi/∂T)P for a liquid corresponds to the state of its molecular force field, while the dynamics of its change follows the evolutional changes in the macroscopic force field of the liquid. This hypothesis allows us to develop a thermodynamic scale of states of the force field of liquids, which is a dimensionless function and is called the index of evolution (Ievol) of the force field of a liquid. The equation of the index of evolution of the force field for all associated liquid-phase systems is given.


 Please wait while we load your content...
Please wait while we load your content...