Turn-on exciplex fluorescence induced by complexation of nonfluorescent pentafluorinated dibenzoylmethanatoboron difluoride with benzene and its derivatives†
Abstract
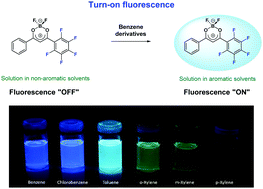
A new pentafluorinated derivative of (dibenzoylmethanato)boron difluoride (5F-DBMBF2) was synthesized and studied as an “off–on” fluorescent probe for benzene derivatives with a reversible detection capability. The structure and optical properties of the DBMBF2 derivative with one pentafluorinated phenyl group were studied by X-ray single-crystal measurements, steady-state UV-visible spectroscopy and computational modeling. 5F-DBMBF2 is non-planar in the ground state, the fluorinated phenyl ring and dioxaborine heterocycle are inclined at an angle of 30° relative to each other. 5F-DBMBF2 shows no detectable fluorescence in non-aromatic solvents, but exhibits exciplex emission in the presence of benzene, chlorobenzene, toluene, o- and m-xylenes with considerable red-shifted maxima as compared with corresponding exciplexes of the parent unsubstituted DBMBF2. DFT calculations predict that the geometric rearrangements during the exciplex formation from a van der Waals complex of 5F-DBMBF2 with benzene in the ground state involve rotating the perfluorinated phenyl ring by about 10°, shortening of the C–C bond between the phenyl and dioxaborine rings, and a decrease in the average interplanar distance between 5F-DBMBF2 and benzene molecules by about 0.7 Å.


 Please wait while we load your content...
Please wait while we load your content...