Magnetic, luminescence, topological and theoretical studies of structurally diverse supramolecular lanthanide coordination polymers with flexible glutaric acid as a linker†
Abstract
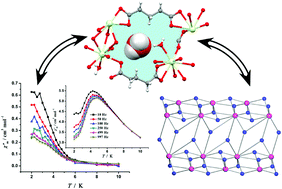
Lanthanide coordination polymers (CPs) have attracted great interest for the development of new functional materials since they possess great structural complexity and a plethora of magneto-optical properties. Hence, herein, we studied the structural, topological, magnetic and optical properties of eight lanthanide CPs based on the very simple ligand glutaric acid (H2Glu):{[Ln(Glu)1.5(H2O)]·xH2O}n; (1), (2), (3) and (4), {[Ln2(Glu)3(DMF)(H2O)]·H2O}n; (5) and (6) and [Ln2(Glu)3(H2O)4]n (7) and (8) [Ln = Tb (1), Sm (2), Eu (3), Gd (4), Dy (5), Pr (6), Eu (7) and Gd (8); Glu = glutarate dianion, DMF = dimethylformamide; x = 2 for CPs 1, 2 and 4 and x = 0 for CP 3]. From a structural point of view, four different coordination modes were noted for the glutarate linker, specifically, (μ2-κ4, η2:η2), (μ3-κ5, η1:η2:η2), (μ4-κ6, η1:η2:η1:η2) and (μ4-κ5, η1:η1:η1:η2). The classification of the geometries of the coordination spheres showed that all the CPs feature muffin geometry with the exception of Dy2, Pr1 and Gd2 in CPs 5, 6 and 8, respectively, which are best described by the spherical monocapped square antiprismatic geometry. The topological simplification and classification of the coordination nets revealed that the topological types are strongly dependent on the metal-to-ion ratio in the resulting CP. The tcs topological type was observed in 1–4, which to date has predominantly appeared in CPs possessing two different ligands. This observation was ascribed to the high coordination number of the metal node based on the analysis of literature precedents. CPs 5 and 6 featured a four-nodal 3,3,3,9-connected net with the {411.616.89}{42.6}{43}{43} point symbol, which is unknown to date. The magnetic studies identified the presence of a strong magnetocaloric effect in the gadolinium CP 8. The value of the volume-normalized entropy parameter −ΔSmaxm was 62.4 mJ cm−3 K−1 at 2 K and ΔH = 7 T. The magnetocaloric effect was much larger than that for most reported Gd3+-based coordination compounds, and it was ascribed to the high magnetic density of the CP. For dysprosium CP 5, the presence of a slow magnetic relaxation process was detected based on the analysis of the frequency-dependent studies. CPs 1 (Tb3+), 2 (Sm3+), 3 and 7 (Eu3+) displayed characteristic f–f photoluminescence in the visible spectral region. Finally, DFT calculations were performed to analyze the hydrogen bonding interactions between the coordinated and non-coordinated water molecules and glutarate-based metallomacrocycles and to quantify the influence of metal coordination on the strength of the hydrogen bonds.


 Please wait while we load your content...
Please wait while we load your content...