Iron-based metal–organic framework as an effective sorbent for the rapid and efficient removal of illegal dyes†
Abstract
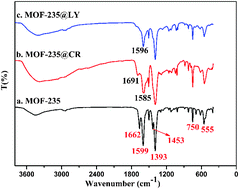
A metal organic framework (MOF-235) was fabricated by a simple solvothermal method and utilized as an adsorbent for the selective removal of electron-rich conjugated dyes. Parameters that influenced the adsorption process were investigated and the excellent adsorption properties of MOF-235 toward Congo red (CR) and lemon yellow (LY) are also exhibited in a wide range of pH 3–9. Moreover, the adsorption kinetics and thermodynamics were determined, indicating that the Langmuir model can well describe the adsorption isotherm and the pseudo-second order model can satisfactorily describe the adsorption kinetics. Thanks to the large hydrophobic effect between electron-deficient MOF and electron-rich conjugated dye molecules, the ultrahigh maximum adsorption capacity for CR and LY calculated by the Langmuir model is up to 1250 and 250 mg g−1, respectively, which surpass that of most reported adsorbents. Notably, the maximum adsorption capacity of adsorption of CR and LY is up to 1131 and 209 mg g−1 in fruit juice, respectively. Additionally, the synthesized MOF-235 has a high adsorptive selectivity and fast adsorption rate and can be regenerated for removal by washing with ethanol containing NaOH solution. These results demonstrated that MOF-235 would be an effective and easily reusable adsorbent for the adsorptive removal of CR and LY from fruit juice.


 Please wait while we load your content...
Please wait while we load your content...