Experimental and computational studies of noncovalent interactions in the metal-free ternary Lys–tn–ATP system†
Abstract
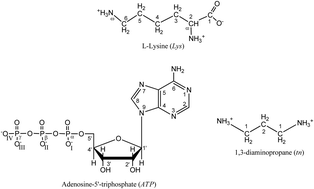
Noncovalent interactions have been studied with the use of experimental (potentiometric and spectroscopic measurements) and computational (molecular modeling and DFT) studies in a lysine, 1,3-diaminopropane and adenosine-5′-triphosphate system. The preferred coordination modes and possible structures of complexes of two obtained species (Lys)H4(tn)(ATP) and (Lys)H3(tn)(ATP) have been determined. Interestingly only the oxygen atoms from the phosphate groups of the nucleotide and the protonated amine groups from the amino acid and polyamine are engaged in the interactions. In the binary adducts there is an involvement of the electron donor endocyclic nitrogen atoms from the ATP purine ring. In contrast, this was not detected in the ternary adduct. The carboxyl group from lysine takes part in the interactions (after deprotonation of the α-amine group) in the adduct (Lys)H3(tn)(ATP), but there is no indication of its engagement in the interactions in (Lys)H4(tn)(ATP). Similar results were obtained in both experimental and computational studies.


 Please wait while we load your content...
Please wait while we load your content...