Interaction of a bioactive molecule with surfaces of nanoscale transition metal oxides: experimental and theoretical studies†
Abstract
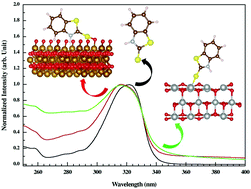
We report a detailed experimental and theoretical investigation on the interaction between the bioactive benzthiazoline-2-thione (BTT) molecule and metal oxide surfaces suspended in aqueous solution. The metal oxides in their nanostructured form were synthesized via sol–gel synthesis. The interactions of BTT with α-Fe2O3 and NiO oxide surfaces were probed by using spectroscopic signatures with a suspension of nanoparticles in aqueous medium. The spectral change of BTT between its free state and bound state onto the suspended metal oxide surfaces in aqueous solution functions as the signature of significant interactions. BTT spectra exhibit a hypsochromic shift of about 4–7 nm when it is attached to both the α-Fe2O3 and NiO oxide surfaces. Density functional theory (DFT) calculations reveal that charge transfer takes place from BTT through the p-orbital of the exocyclic sulphur atom to the O-p dominated conduction band of α-Fe2O3 (O-terminated) and to the Ni-3d dominated valence band of the NiO surfaces. This extension of charge from BTT to the metal oxide surfaces is attributed as the origin of the hypsochromic shift in the UV-Vis spectra of the BTT molecule.


 Please wait while we load your content...
Please wait while we load your content...