Molecular design and selection of 1,2,5-oxadiazole derivatives as high-energy-density materials†
Abstract
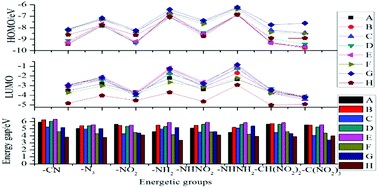
In the present study, 64 energetic compounds based on 1,2,5-oxadiazole were designed and their energy gaps, heats of formation (HoF), detonation properties, and thermal stabilities were fully investigated by density functional theory at the B3LYP/6-311G(d,p) level. All the designed compounds possessed high positive ΔHf,gas values and the –NH– bridge and –N3 group were the most effective ones for improving the HoFs of these compounds. The predicted densities and detonation properties showed that –C(NO2)3 and –O– were the most effective groups to improve the densities, while –C(NO2)3 and –NH– were the most effective groups to improve the detonation velocities. When the compounds were substituted by –NHNH2/–NHNO2/–C(NO2)3/–CH(NO2)2 groups, the BDE values of the NH–NH2/NH–NO2/C–(NO2)3/CH–(NO2)2 bonds were the lowest, which indicated that the NH–NH2/NH–NO2/C–(NO2)3/CH–(NO2)2 bond cleavages were the possible thermal decomposition path for these compounds. In addition, incorporating the –NHNH– bridge into the compounds decreased the BDE values. Overall, the effects of the substituents on these properties were combined with those of the bridge groups. Taking the detonation properties and thermal stabilities into consideration, eight compounds (A3, C2, C3, C6, D3, G3, H3, and H5) were selected as high-energy-density compounds and their electronic structures were investigated to gain a better understanding of these compounds.


 Please wait while we load your content...
Please wait while we load your content...