Li3Ba4Sc3(BO3)4(B2O5)2: featuring the coexistence of isolated BO3 and B2O5 units†
Abstract
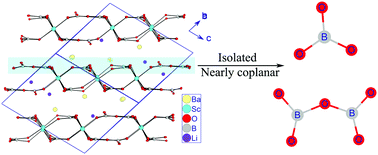
A new mixed alkali/alkaline earth scandium borate with the composition Li3Ba4Sc3B8O22, has been obtained using a high-temperature flux method. Its structure contains two types of isolated and almost co-planar BO3 and B2O5 groups and can be described as Li3Ba4Sc3(BO3)4(B2O5)2. Sc atoms connect the oxygen atoms belonging to the BO3 or B2O5 groups in the form of six-coordinated octahedron ScO6 to compose a unique 2∞[Sc3B8O22] double-layer with the Li and Ba atoms filling in the space among the double layers. Although many kinds of B–O groups have been found in borates, two types of isolated B–O groups coexisting in a single compound is uncommon as it directly violates the Pauling rule of parsimony.


 Please wait while we load your content...
Please wait while we load your content...