The role of a weakly coordinating thioether group in ligation controlled molecular self-assemblies and their inter-conversions in Ni(ii) complexes of l-methionine derived ligand†
Abstract
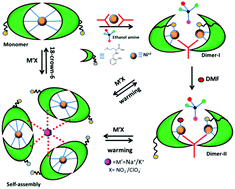
A series of Ni(II) complexes of an L-methionine derived reduced Schiff base ligand has been synthesized and their interconversions are investigated for the first time with the help of a variety of physico-chemical techniques. The different reactant/reagent conditions lead to mono- (1), di- (2a/2b) and tri-nuclear (3a/3b) Ni(II) complexes and they are unequivocally characterized by single crystal X-ray crystallography. Alkali ions K+/Na+ can serve as efficient templates for the conversion of 1 to tri-nuclear complexes 3a/3b which are thermally stable at increased temperature of 70 °C, but amenable to dissociation to 1 in the presence of strongly interacting 18-crown-6-ether. 2a gets transformed to 2b in DMF, in which the essential structural change is the incorporation of one DMF molecule to the coordination sphere of one of the Ni(II) centers with concomitant release of the coordinating thioether arm of the ligand. 2a and 2b can also be converted to a self-assembled complex (3a/3b) by alkali metal salt addition at ambient temperature. Warming the solution to 70 °C disassembles the tri-nuclear complexes with a perceptible colour change and this cycle can be repeated several times. Additional evidence for the inter-conversion and retention of all the complex species in solution is clearly established by ESI-mass and UV-vis spectroscopy. Variable temperature magnetic analysis shows weak ferromagnetic and antiferromagnetic coupling for binuclear and self-assembled complexes, respectively.


 Please wait while we load your content...
Please wait while we load your content...