New methodology of nucleophilic substitution at three different centers of a seleniranium intermediate in reactions of 2-bromomethyl-1,3-thiaselenole with mercapto benzazoles†
Abstract
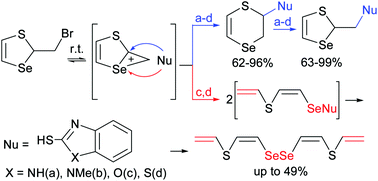
A new methodology of nucleophilic substitution at three different centers of a seleniranium intermediate in reactions of 2-bromomethyl-1,3-thiaselenole with mercapto benzazoles containing various combinations of heteroatoms (N, O and S) was developed. The reactions were accompanied by rearrangement with ring extension affording a new family of 2,3-dihydro-1,4-thiaselenins functionalized with benzazoles in high yields. The latter compounds in turn underwent rearrangement with ring contraction via an intermediate seleniranium cation giving new 1,3-thiaselenole ensembles in high yields. The reaction of 2-bromomethyl-1,3-thiaselenole with 1,3-benzothiazole-2-thiol proceeded in a stereoselective manner leading to (Z,Z)-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSCH
CHSCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSeSeCH
CHSeSeCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHSCH
CHSCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 as the result of nucleophilic attack at the selenium atom of the seleniranium intermediate with ring opening followed by disproportionation.
CH2 as the result of nucleophilic attack at the selenium atom of the seleniranium intermediate with ring opening followed by disproportionation.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...