Structures of solvated ferrous ion clusters in ammonia and spin-crossover at various temperatures†
Abstract
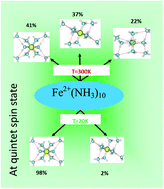
Iron plays a key role in the evolution of living systems and so it is an essential element in a wide range of biological phenomena, energy transduction mechanisms, and oxygen carriers. In this work, we investigated various isomers of the Fe2+(NH3)n=1–10 complex in the singlet, triplet and quintet spin states in the gas phase and their relative stabilities in a wide range of temperatures (0 K and 25–400 K). All the calculations are performed at the M06-2X/6-31++G(d,p) and MP2/6-31++G(d,p) levels of theory. It emerges from this work that although M06-2X could not in all cases be suitable for investigating the structural parameters of this complex in the singlet spin state, it gives excellent relative energies as compared to MP2 results and therefore could be recommended for evaluating the solvation free energy of the ferrous ion rather than the time consuming MP2 method. In addition, with a few exceptions, structures obtained at the MP2 and M06-2X levels of theory are very similar. The solvated ferrous ion in the singlet, triplet and quintet spin states in ammonia is hexa-coordinated, irrespective of the temperature. This result slightly contrasts the reported finding concerning the hydrated ferrous ion for which the CN is ∼6 around 215 K and less at a higher temperature of 305 K. Furthermore, the quintet spin state structures dominate exclusively lower spin state ones at all temperatures in such a way that no natural spin-crossover is possible between the quintet spin state and the lower spin states in the clusters of Fe2+(NH3)n=1–10. However, the spin-crossover is naturally possible around 200 K between the singlet and the triplet spin states for n ≥ 6, and the latter is preferred at high temperatures while the former is preferred at lower temperatures. We also pointed out that the available clustering energies at modest or higher cluster sizes (n > 7) for solvated metal ions neither depend on the spin state of the metal, or on the type of metal (alkaline earth, transition metals,…), or on the ligand type (ammonia, water,…). Thus, the solvation energies of metal ions did not depend on their spin states. We also anticipated that the solvation free energy or enthalpy of the ferrous ion in ammonia could be determined by clustering up to n = 9 or 10.


 Please wait while we load your content...
Please wait while we load your content...