Mechanofluorochromic behaviors of triphenylamine functionalized salicylaldimine difluoroboron complexes†
Abstract
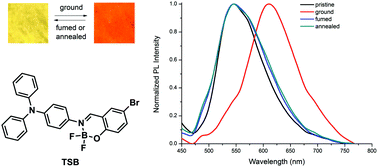
Triphenylamine functionalized salicylaldimine difluoroboron complexes bearing different substituents have been synthesized. The photophysical results showed that these complexes exhibited intramolecular charge transfer (ICT) emission and solvatochromism. The fluorescence of these complexes could be well-tuned by the substituents in the ligands. The introduction of tert-butyl groups causes the complex TST to show obvious aggregation-induced emission (AIE) activity. In particular, the complexes without other substitutions or with the substitutions of bromo, tert-butyl and methoxyl on salicylaldimine moieties displayed reversible mechanofluorochromic (MFC) behaviors. XRD patterns and DSC curves of the complexes in different solid states suggested that the transformation between crystalline and amorphous states was responsible for the MFC properties. The relationship between the molecular structures and MFC properties suggested that the high polarity of the conjugated skeletons in difluoroboron complexes was beneficial for realizing high-contrast performance in mechanofluorochromism.


 Please wait while we load your content...
Please wait while we load your content...