Synthesis, structure and magnetic properties of multipod-shaped cobalt ferrite nanocrystals†
Abstract
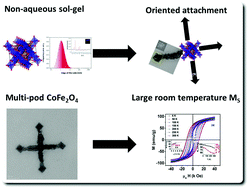
We applied a general non-aqueous route for the formation of mono-, bi-, tri-, tetra, hexapod and multipod magnetic spinel ferrite metal oxide (CoFe2O4) nanocrystals. The magnetic CoFe2O4 nanocrystals were characterized by X-ray diffraction, high-resolution transmission electron microscopy, Raman spectroscopy, magnetometry and Mössbauer spectroscopy, to understand their structure and magnetic properties. These CoFe2O4 nanoparticles have the smallest particle size reported ever, with a mean diameter of ∼3 nm. The coercivity was found to be larger compared to that reported in the literature for spherical particles. Mössbauer spectroscopy indicated a magnetic transition above room temperature, where 20% of the Fe ions transit to a paramagnetic state, around 400 K. From the thermal dependence of magnetic parameters, the blocking temperature was estimated to be around 425 K. These studies indicate that our CoFe2O4 nanocrystals are different from their cubic/spherical counterparts, which generally display a single domain character. These CoFe2O4 nanocrystals display a strong shape anisotropy dependent magnetic property.


 Please wait while we load your content...
Please wait while we load your content...