Creation of thio and selenocyanate derivatives of 4-quinolone via regioselective C–H bond functionalization under ambient conditions†
Abstract
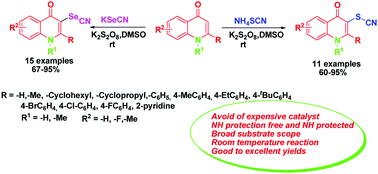
An operationally simple C–SCN and C–SeCN bond formation technique to generate different SCN/SeCN substituted 4-quinolone derivatives using NH4SCN/KSeCN in excellent yields was developed. The versatility of this protocol has successfully been demonstrated by extension of the suitable reaction conditions to substrates having different substituents. This regioselective C–H bond functionalization approach provides direct access to structurally diverse 3-thiocyanated/selenocyanated 4-quinolone derivatives. Moreover, this new method does not require any transition metal catalyst and pre-requisite –NH protection of 4-quinolone derivatives. Antimicrobial effects (zone inhibition test and MIC analysis) were determined with both SCN/SeCN substituted 4-quinolone derivatives.


 Please wait while we load your content...
Please wait while we load your content...