Aluminium complex-catalysed hydroboration of alkenes and alkynes†
Abstract
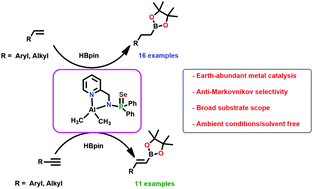
We demonstrate an efficient method for the hydroboration of terminal alkenes or alkynes with pinacolborane (HBpin) using the aluminium catalyst, [κ2-{Ph2P(=Se)NCH2(C5H4N)}Al(CH3)2] (1), supported by a functionalized amidophosphine ligand under mild and solvent-free conditions to afford the corresponding alkyl and alkenyl boronate esters in high yield. This protocol involves the chemoselective formation of an anti-Markovnikov product exclusively. Both terminal alkenes and alkynes bearing a wide array of electron-withdrawing and donating functional groups can easily be converted to the corresponding product through the formation of aluminum hydride as an active catalytic species.


 Please wait while we load your content...
Please wait while we load your content...