Synthesis and characterization of analogues of glycine-betaine ionic liquids with the 4-chlorosalicylate anion and their use in the extraction of copper(ii) ions
Abstract
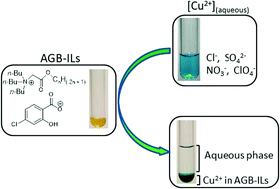
A series of 5 analogues of glycine-betaine based ionic liquids (AGB-ILs) viz. [tri(n-butyl)(2-alkoxy-2-oxoethyl)]ammonium (alkoxy = n-butoxy, n-pentoxy, n-hexoxy, n-heptoxy and n-octoxy) associated with the 4-chlorosalicylate anion have been synthesized and fully characterized by spectroscopic methods (IR, 1H and 13C NMR) and elemental analysis. The effect of the alkyl chain length of the cation core on the physicochemical properties of these ionic liquids has been investigated. The photoluminescence study of these AGB-ILs shows that the nature of the organic cation does not have a great influence on their fluorescence induced by the 4-chlorosalicylate anion. The extraction ability of these ionic solvents towards different aqueous copper(II) salts was investigated. It was found that the copper extraction yield with these AGB-ILs depends not only on the length of the alkyl chain (the shorter the alkyl chain, the higher the extraction yield) but mostly on the nature of the counter-anion of the copper(II) salt; this extraction yield decreases in the order ClO4− > NO3− ≫ Cl− > SO42−. The mechanism studied shows that ion pairing extraction is especially favoured by AGB-ILs possessing long alkyl chains.


 Please wait while we load your content...
Please wait while we load your content...