A theoretical study of adsorbed non-metallic atoms on magnesium chloride monolayers†
Abstract
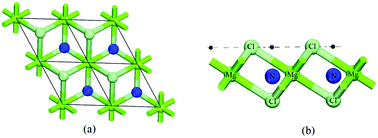
A detailed study involving non-metallic atoms (boron, nitrogen and carbon atoms) adsorbed on magnesium chloride (MgCl2) monolayers has been performed by density functional theory. The obtained results demonstrate that these dopant atoms present three different stable configurations in relation to the reference plane formed by the chlorine atoms. For each stable configuration the MgCl2 monolayer, electronic and structural properties undergo significant changes depending on whether the dopant atom assumes a tetrahedral or octahedral configuration in relation to the first neighbors of the chlorine and magnesium atoms. These changes happen due to the presence of energy levels related to the dopant atoms in the Fermi level region. These features suggest the possibility of optoelectronic applications for the MgCl2 monolayer doped with non-metallic atoms. In addition, the calculated adsorption energies indicate that the structural site used for adsorption of the boron, nitrogen and carbon atoms can be efficiently used to capture other atomic or molecular systems.


 Please wait while we load your content...
Please wait while we load your content...