A highly selective and sensitive ESIPT-based coumarin–triazole polymer for the ratiometric detection of Hg2+†
Abstract
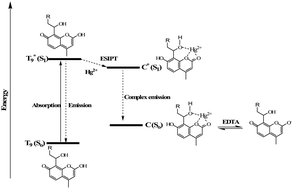
A novel fluorescent polymer for Hg2+ recognition was developed based on the phototautomerism mechanism of 7-hydroxycoumarin in aqueous solution. The developed system showed higher sensitivity and specificity towards Hg2+ in the acetonitrile/water mixture over various other metal ions, with a significant fluorescence quenching of the main emission band. It was shown that the presence of Hg2+ affects the chemical equilibrium of the 7-hydroxycoumarin phototautomers through the excited state intramolecular proton transfer (ESIPT) mechanism, which results in ratiometric fluorescence response upon stepwise addition of Hg2+. The occurrence of ESIPT mechanism was explained based on a comparison of the 1H NMR spectra of the starting alkynylated coumarin (4) in a non-polar aprotic solvent (CDCl3) and in a polar aprotic solvent (DMSO-d6). This was further confirmed by 1H NMR of 4 upon stepwise addition of Hg2+ in a polar aprotic solvent (DMSO-d6). From titration experiments, a higher sensitivity with a detection limit at the nanomolar level (50 nM) was observed and the response was fast and reversible. The real applications of the developed system were investigated in real samples and satisfactory results were obtained for both fresh and lake water samples.


 Please wait while we load your content...
Please wait while we load your content...