Production of propylene glycol (1,2-propanediol) by the hydrogenolysis of glycerol in a fixed-bed downflow tubular reactor over a highly effective Cu–Zn bifunctional catalyst: effect of an acidic/basic support†
Abstract
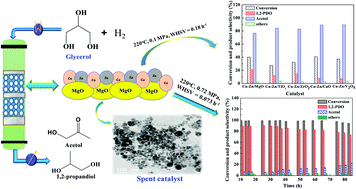
In this study, different acidic (V2O5, ZrO2, and TiO2) and basic (CaO and MgO) oxide-supported copper–zinc bimetallic catalysts were prepared by a deposition–precipitation method, and they were evaluated for the vapor-phase hydrogenolysis of glycerol to propylene glycol at 0.1 MPa and at 220 °C. The catalysts were thoroughly characterized by different techniques such as BET, XRD, H2-TPR, NH3 and CO2 TPD, N2O adsorptive decomposition, TEM, XPS, FE-SEM and TGA. Among all the supported catalysts, the Cu–Zn/MgO catalyst was found to be the most selective to propylene glycol. High copper-metal dispersion (∼5%), surface area (∼23 m2 g−1), the highest basicity (0.25 mmol CO2 g cat−1) and the availability of partially reduced copper species (Cu2O, CuO and Cu0) were the primary reasons for the higher propylene glycol selectivity. At optimum reaction conditions, i.e., at 220 °C, 0.72 MPa and a weight hourly space velocity (WHSV) of 0.073 h−1, ∼98.5% conversion of glycerol with ∼89% selectivity to propylene glycol was obtained over the Cu–Zn/MgO catalyst. This catalyst was also found to be stable for a long period of time (84 h) without much deactivation and without decrease in the selectivity to propylene glycol.


 Please wait while we load your content...
Please wait while we load your content...