Metal free, facile sulfenylation of ketene dithioacetals catalyzed by an HBr–DMSO system†
Abstract
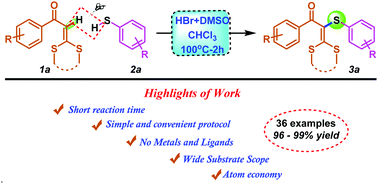
A transition metal free, highly efficient, sulfenylation of ketene dithioacetals catalyzed by an HBr–DMSO system is achieved. This strategy employs inexpensive and readily available HBr and DMSO to provide a direct C–H bond sulfenylation with a broad range of aryl thiols. Highlights of the present strategy are convenient, straightforward approach, metal free, short reaction time and excellent yields. This sulfenylated product is also successfully transformed for the synthesis of pyrazole derivatives in excellent yields.


 Please wait while we load your content...
Please wait while we load your content...