Structural and electronic properties of MB22− (M = Na, K) clusters: tubular boron versus quasi-planar boron forms†
Abstract
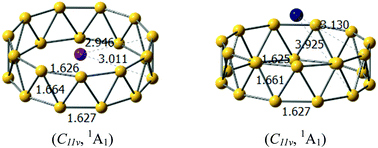
Electron deficiency of boron atom has led to the abundant chemical properties of boron clusters, such as intriguing structures, unique multi-center bonding and electronic properties, as well as the structural evolution from planar to three-dimensional forms. Tubular boron clusters or all-boron fullerenes serve as an important indicator for the structural evolution of boron clusters. Moreover, tubular boron clusters can be considered as precursors for boron nanotubes. However, the anionic tubular boron clusters have been theoretically identified to be less stable in comparison to the planar or quasi-planar forms, which is clearly disadvantageous for the gas-phase experimental characterization. In this study, we follow the strategy that the stability of the tubular boron clusters can be significantly enhanced by doping alkali metals, suggesting MB22− (M = Na, K) as the largest anionic boron nanotube predicted until now. The title tubular boron clusters were more stable than the quasi-planar forms because of the double aromaticity with the delocalized π + σ electrons in boron motifs as well as the optimal electrostatic interactions between the cationic M+ and anionic tubular boron motifs, originating from the strong charge transfer from the alkali metals to the boron motifs.


 Please wait while we load your content...
Please wait while we load your content...