Do An(iii) and Ln(iii) ions form heteroleptic complexes with diglycolamide and hydrophilic BT(B)P ligands in solvent extraction systems? A spectroscopic and DFT study†
Abstract
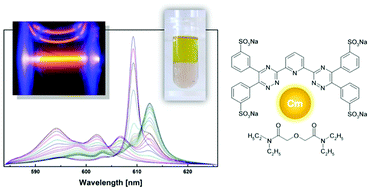
Time-Resolved Laser Fluorescence Spectroscopy (TRLFS) was used to study the complexation of Cm(III) and Eu(III) in solvent extraction systems consisting of an organic phase containing TODGA (tetra-n-octyl diglycolamide) as an extracting agent and an aqueous phase containing hydrophilic complexing agents, tetrasodium salts of 2,6-bis(5,6-di(3-sulphophenyl)-1,2,4-triazin-3-yl)pyridine, SO3-Ph-BTP4−, or 6,6′-bis(5,6-di(3-sulphophenyl)-1,2,4-triazin-3-yl)-2,2′-bipyridine, SO3-Ph-BTBP4−. Only one complex, homoleptic [Cm(TODGA)3](NO3)3, was found in the organic phase. No heteroleptic complexes of Cm(III) with TODGA and SO3-Ph-BTP4− have been detected. In contrast, in dilute aqueous HClO4 solutions containing TEDGA (a hydrophilic homologue of TODGA) and SO3-Ph-BTP4− ligands, seven different Cm(III) species were identified by their distinct emission spectra. Apart from the emission bands corresponding to the hydrated Cm3+ cation and to the complexes [Cm(SO3-Ph-BTP)]− and [Cm(TEDGA)n]3+ (n = 1–3), two further emission bands were ascribed to heteroleptic complexes [Cm(TEDGA)(SO3-Ph-BTP)]− and [Cm(TEDGA)2(SO3-Ph-BTP)]−. In a similar aqueous solution containing TEDGA and SO3-Ph-BTBP4−, only one heteroleptic complex was identified, [Cm(TEDGA)(SO3-Ph-BTBP)]−. In addition, quantum chemical optimisation of the structures of the postulated heteroleptic complexes was performed.


 Please wait while we load your content...
Please wait while we load your content...