Abstract
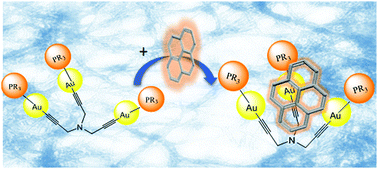
The synthesis of three gold(I) tripodal complexes derived from tripropargylamine and containing the water soluble phosphines PTA (1,3,5-triaza-7-phosphaadamantane), DAPTA (3,7-diacetyl-1,3,7-triaza-5-phosphabicyclo[3.3.1]nonane) and TPPTS (triphenylphosfine-3,3′,3′′-trisulfonic acid trisodium salt) is described here. The three complexes are observed to give rise to the formation of supramolecular aggregates in water and very long fibers. This property has been analyzed by means of 1H-NMR spectroscopy at different concentrations and SAXS. The results point out the important role of the phosphine moieties as the main enthalpic or entropic contribution in the resulting Gibbs energy of aggregate formation. The tripodal structure of the three complexes together with the presence of gold(I) atoms makes them ideal candidates to interact with hydrophobic molecules also in water. For this, the interaction with pyrene in this solvent has been evaluated with successful results in all three complexes. The highest association constant corresponds to 2 as the host. DFT studies indicate the location of pyrene in the tripodal cavity as the most stable conformation. The interaction with pyrene has been additionally studied within cholate hydrogel matrixes pointing out the stability of the resulting host:guest adducts in different media.


 Please wait while we load your content...
Please wait while we load your content...