Selective oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran over a Cu–acetonitrile complex†
Abstract
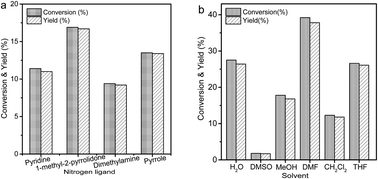
Herein, Cu(NO3)2/acetonitrile was proven to be an efficient oxidation system in the oxidation of 5-HMF to DFF in an aqueous solution. After optimization of the reaction conditions, the DFF yield of 97.0% was obtained under the conditions of 1.5 mmol 5-HMF, 1.5 mmol Cu(NO3)2, 0.48 ml acetonitrile, 10 ml H2O, 80 °C, and 18 h. The oxidation system could be recycled at least three times by adding HNO3, which avoided the risk of production of Cu-containing waste. The formation of Cu(CH3CN)4+ in the aqueous solution played an important role in enabling the oxidation of 5-HMF via the redox cycle of Cu2+/Cu+. To further improve the reaction efficiency, a reactive-extraction process was developed by introducing toluene as the organic phase due to its excellent extraction capacity for DFF. As a result, the reaction time was shortened to 8 h, and the highest DFF yield of 98.9% was achieved.


 Please wait while we load your content...
Please wait while we load your content...