Assembly of three pharmaceutical salts/cocrystals of tetrahydroberberine with sulfophenyl acids: improving the properties by formation of charge-assisted hydrogen bonds†
Abstract
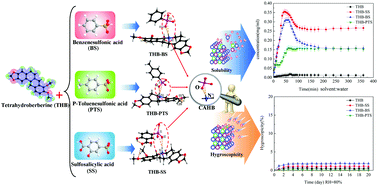
With our ongoing purpose to further explore the role of charge-assisted hydrogen bond (CAHB) recognition in synthetic processes of pharmaceutical salts/cocrystals and in the aspect of improving physicochemical properties of tetrahydroberberine (THB), three new pharmaceutical salts/cocrystals of THB with benzenesulfonic acid (C20H22NO4·C6H5O3S, THB-BS), p-toluenesulfonic acid (C20H22NO4·C7H7O3S·CH4O, THB-PTS) and sulfosalicylic acid (C20H22NO4·C7H5O6S, THB-SS) have been designed and synthesized. To the best of our knowledge, these three pharmaceutical salts/cocrystals of THB represent the first exploration in which sulfophenyl acids are introduced into salts/cocrystals of THB to improve the physicochemical properties of THB. As expected, the solubility of THB is dramatically improved in pure water by forming pharmaceutical salts/cocrystals. Astoundingly, the hygroscopic stabilities of the three pharmaceutical salts/cocrystals of THB are also nearly unchanged in comparison with pure THB. Preliminary analyses of the relationship between structures and hygroscopic stabilities indicate that it may be difficult to combine the sulfonic groups of sulfophenyl acids with water molecules because the hydrogen bonds between the sulfonic groups and their adjacent groups are closed to saturation. Further analyses revealed that the molecular recognition of CAHBs (N+–H⋯O−) between hydrogen proton donors (sulfophenyl acids) and acceptors (THBs) could stabilize crystal packing of pharmaceutical salts/cocrystals of THB, and CAHBs are typically strong intermolecular interactions. This successful work implies that molecular recognition of CAHBs plays a key role in tuning the style of molecular packing to improve the physicochemical properties of active pharmaceutical ingredients, and it provides a facile route for designing and synthesizing novel salts/cocrystals of clinical medicine of THB on molecular length scales.


 Please wait while we load your content...
Please wait while we load your content...