A straightforward and versatile FeCl3 catalyzed Friedel–Crafts C-glycosylation process. Application to the synthesis of new functionalized C-nucleosides†
Abstract
A new, straightforward and eco-compatible route to C-(hetero)aryl nucleosides is reported. This ribosylation process consists of a one-step FeCl3 catalyzed Friedel–Crafts β-C-glycosydation reaction. This reaction occurs through an oxonium intermediate from readily available protected sugars leading to functionalized C-nucleosides with high stereocontrol. The expected new C-nucleosides were obtained in good yields within a short reaction time (10 min). Moreover, this approach is compatible with the use of a range of bulky (poly)aromatics such as naphthalene, anthracene and pyrene, which are not easily accessible via classical routes. These new C-aryl-nucleosides exhibit interesting photophysical properties, underling their potential use for further nucleic acid labelling.
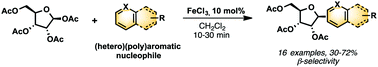


 Please wait while we load your content...
Please wait while we load your content...