Catalytic activity of a new Ru(ii) complex for the hydrogen transfer reaction of acetophenone and N-benzylideneaniline: synthesis, characterization and relativistic DFT approaches†
Abstract
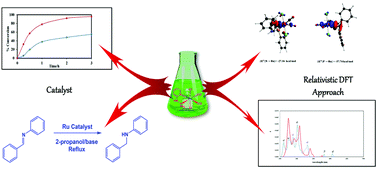
The synthesis and characterization of a new ruthenium(II) complex containing a hemilabile P^N-ligand are reported. The catalytic activity of this complex was evaluated in the hydrogen transfer reaction of acetophenone and N-benzylideneaniline achieving conversions around 35 and 96% respectively. Condensed Fukui functions in a relativistic DFT scheme show that the regions for the electrophilic (f−) and nucleophilic (f+) attack are Cl− in the axial position, regions belonging to the P^N ligand and the metal center. The lability of the ligands is determined using different theoretical approaches like Morokuma–Ziegler energy decomposition analysis combined with the Extended Transition State with Natural Orbitals of Chemical Valence (ETS-NOCV). The major lability for the –Cl ligand (protic environment) is ideal for designing a new ruthenium hydride complex, suitable for catalytic applications. The σ-donation determines the degree of covalence of the chemical bond involving a lower trend to dissociate, as is the case of the P^N-ligand. The most important excitation energies appear in the region between 250 and 410 nm and are assigned to ligand to ligand charge transfer (LLCT) and metal to ligand charge transfer (MLCT) transitions.


 Please wait while we load your content...
Please wait while we load your content...