Multi-gram scale synthesis of a bleomycin (BLM) carbohydrate moiety: exploring the antitumor beneficial effect of BLM disaccharide attached to 10-hydroxycamptothecine (10-HCPT)†
Abstract
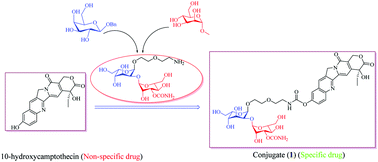
The “tumor-seeking” role of bleomycin (BLM) disaccharide has been demonstrated to serve as a promising tool for cancer diagnosis and a potential ligand for targeted therapy. However, these practical applications are often hampered by the lack of BLM disaccharide. Herein, an efficient multi-gram synthesis of peracetylated BLM disaccharide 20 is achieved by a TMSOTF-mediated glycosidation coupling manner in 43.6% overall yield in terms of benzyl galactoside. The critical innovation of the synthetic strategy is that inexpensive benzyl galactoside was first adopted to prepare an L-gulose subunit 3 as a glycosyl acceptor, with a much shorter route in 73.0% yield, and a 3-O-carbamoyl-mannose donor 4 was achieved in 47.2% yield by lowering the amount of dibutyltin oxide, and merging aminolysis and selective deacetylation into a one-pot reaction. Next, the incorporation of BLM disaccharide into 10-hydroxycamptothecin (10-HCPT), a non-specific model compound, to form conjugate 1 could significantly improve the antitumor activity and display obvious selectivity toward cancerous and normal cells in comparison with 10-HCPT. Moreover, BLM disaccharide itself was non-cytotoxic, clearly indicating the importance and potential of BLM disaccharide in solving the targeted antitumor therapy of cytotoxic drugs.


 Please wait while we load your content...
Please wait while we load your content...