The facile hydrothermal synthesis of CuO@ZnO heterojunction nanostructures for enhanced photocatalytic hydrogen evolution†
Abstract
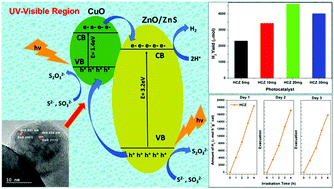
In this work, we report a simple synthesis process for the construction of CuO@ZnO p–n heterojunctions via the in situ deposition of p-type CuO nanoparticles on the surface of three dimensional ZnO. In this study, a CuO@ZnO heterojunction material is obtained via an effective hydrothermal method. At the interface of the CuO@ZnO p–n heterojunctions, due to a difference in Fermi levels and suitable band position, the transfer of electrons and holes across the p–n heterojunction interface can be easily achieved, leading to improved photogenerated charge carrier dynamics. The photocatalyst exhibited high photocatalytic activity and long-term stability towards photochemical hydrogen production and the reduction of a pollutant, methylene blue (MB) dye, in solution. XRD showed the existence of CuO in the sample with the peak at 38.7° corresponding to the (111) plane. XPS analysis also confirmed the presence of a Cu2+ state and the non-existence of metallic Cu and Cu+ states. The photocatalytic activity was evaluated with respect to the liquid-phase degradation of MB at pH values of 3, 7 and 10 under UV-visible light irradiation. The rate of H2 production was high for 20 mg of ZnO and CuO@ZnO under solar irradiation: 2353 and 4604 μmol h−1 g−1, respectively, with the sacrificial reagent Na2S–Na2SO3. This result is competitive with the values obtained with previously reported copper based ZnO photocatalysts for photocatalytic hydrogen production and indicates that further performance improvements could be achieved with an ordered nanostructure morphology. The high photocatalytic H2 production activity is attributed predominantly to the presence of CuO species and the small size of the heterojunction between CuO and ZnO, facilitating interfacial charge carrier transfer from the ZnO nanoparticles. This work reveals the potential of the CuO@ZnO photocatalyst for efficient hydrogen evolution from water splitting and for environmental applications.


 Please wait while we load your content...
Please wait while we load your content...