A Zn based metal organic framework as a heterogeneous catalyst for C–C bond formation reactions†
Abstract
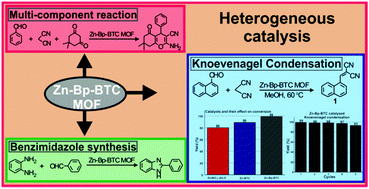
Herein, we report the synthesis and application of a Zn-Bp-BTC MOF (Bp – 4,4′-bipyridine; BTC – 1,3,5-benzene tricarboxylic acid; MOF – metal organic framework) as a heterogeneous catalyst for mediating organic reactions. Initially, conditions were optimized for the Knoevenagel condensation reaction using Zn-Bp-BTC as a heterogeneous catalyst by varying the solvent, temperature and catalyst loading. Although the reaction proceeded at room temperature using methanol as the solvent, 60 °C offered the best yield in a shorter duration. Under optimized reaction conditions, a wide range of α,β-unsaturated dicyano compounds were prepared from the corresponding carbonyl precursor and malononitrile, the active methylene counterpart. Systematic investigation was also carried out to assess the role of the ligand and metal salt in the Knoevenagel condensation reaction. It was found that the Zn-Bp-BTC MOF catalyzed the reaction efficiently in comparison to its analogue Zn-BTC MOF and precursor Zn(NO3)2·6H2O. Finally, catalytic recycling and stability studies showed that the catalyst is able to mediate the reaction for up to five consecutive cycles without undergoing any significant chemical or morphological changes. Further, the catalyst was tested for its efficacy in a multi-component reaction (MCR). A MCR with the Zn-Bp-BTC MOF as the catalyst afforded good yields and there was no reaction in the absence of the catalyst. Similarly, the catalyst was tested for its efficiency in benzimidazole synthesis.


 Please wait while we load your content...
Please wait while we load your content...