Water-soluble binary and ternary palladium(ıı) complexes containing amino acids and intercalating ligands: synthesis, characterization, biomolecular interactions and cytotoxicities†
Abstract
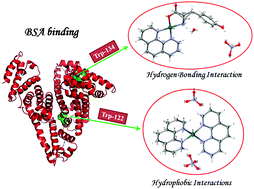
In this study, six water soluble cationic binary (1–3) palladium(ıı) complexes of the type [PdA2](NO3)2(n − 1)H2O and ternary (4–6) palladium(ıı) complexes of the type [PdA(amino acid)](NO3)(n − 1)H2O, where A is 1,10-phenanthroline (phen) (1, 4, 6), 5-methyl-1,10-phenanthroline (5-mphen) (2, 5), and 3,4,7,8-tetramethyl-1,10-phenanthroline (tmphen) (3) and the amino acid is L-tyrosine (tyr) (4, 5) and glycine (gly) (6), have been synthesized and characterized using CHN elemental analyses, ATR-FT-MIR, 1H NMR and 13C NMR and single-crystal X-ray diffraction techniques. The interaction of the complexes with calf thymus DNA (CT-DNA) was investigated using electronic absorption spectral, ethidium bromide (EB) and Hoechst 33258 (HO) displacement assays and thermal denaturation. The pUC19 DNA cleavage activity of the complexes was investigated in the absence and presence of external agents by agarose gel electrophoresis. Quenching of the tryptophan and tyrosine residues of bovine serum albumin (BSA) by the complexes was found to be static. Furthermore, the in vitro cytotoxic effect of the complexes was examined on human tumor cell lines (Caco-2, A549, and MCF-7) and healthy cells (BEAS-2B). All the complexes exhibited different cytotoxic activities.


 Please wait while we load your content...
Please wait while we load your content...