Novel herbicide ionic liquids based on nicosulfuron with increased efficacy†
Abstract
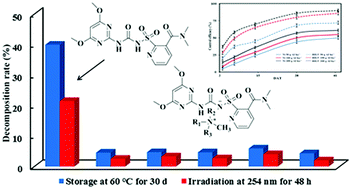
Nicosulfuron is widely used in agriculture because of its high selectivity, wide weeding spectrum, and excellent herbicide performance. However, it is necessary to add adjuvants to stabilize nicosulfuron so as to maximize its weed-control efficacy. In this study, five novel herbicidal ionic liquids (HIL1-5) based on nicosulfuron were synthesized through an acid–base reaction by pairing with tetrabutylammonium, benzyltriethylammonium, 2-hydroxyethyltrimethylammonium, hexadecyltrimethylammonium, and hexadecypyridinium, representing different types of cations with short-chain branched hydrocarbon, long-chain branched hydrocarbon, fused heterocycle, and aromatic hydrocarbon, respectively. The results showed that HILs had a low solubility and surface tension, and moderate octanol–water partition coefficient, which could reduce the risk of leaching and polluting the water environment to some extent. Nicosulfuron-based herbicidal ionic liquids were more stable than the nicosulfuron under high temperature and ultraviolet radiation, and the stability of the herbicidal ionic liquids followed the order of HIL5 > HIL1 > HIL3 > HIL2 > HIL4. HIL5 without any adjuvants exhibited a better control efficacy against gramineous and broadleaf weeds than nicosulfuron. The results showed that the physicochemical property of herbicide could be optimized by selecting suitable paired ions to solve the problems existing in practical application of the herbicide and could reduce its negative impact on the environment.


 Please wait while we load your content...
Please wait while we load your content...