Tuning alkynyl-extended 9,10-dihydroanthracene-based systems into aggregation-induced emission (AIE) luminophores†
Abstract
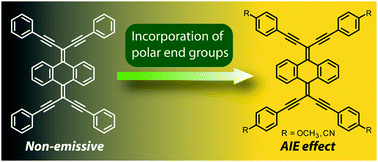
A series of 9,10-bis(1,5-diphenylpenta-1,4-diyn-3-ylidene)-9,10-dihydroanthracenes (BDPPD-DHAs) carrying polar organic functional groups was synthesized through Sonogashira cross-coupling reactions. The structural and electronic properties of these BDPPD-DHA derivatives were investigated by single crystal X-ray diffraction, UV-Vis absorption, and fluorescence spectroscopic analyses. It was found that the incorporation of polar substituents into the BDPPD-DHA backbone could enhance intermolecular attractions and hence significantly affect the properties in the aggregated states. Of particular interest is the observation that OMe and CN-substituted BDPPD-DHAs show aggregation-induced emission (AIE) effects due to restricted intramolecular motions. Our study herein discloses a new venue to develop AIE luminogens by means of rational molecular tailing.


 Please wait while we load your content...
Please wait while we load your content...