Design, synthesis, antifungal activity and 3D-QSAR study of novel pyrazole carboxamide and niacinamide derivatives containing benzimidazole moiety†
Abstract
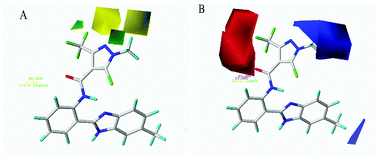
A series of novel pyrazole carboxamide and niacinamide derivatives containing a benzimidazole moiety were designed and synthesized as antifungal candidate agents. All target compounds were characterized by FTIR, 1H NMR, 13C NMR, HRMS and elemental analysis techniques. The structure of compound T1 was further confirmed by single crystal X-ray diffraction analysis. The antifungal activities of the target compounds were evaluated in vitro against four phytopathogenic fungi (namely Botrytis cinerea, Rhizoctonia solani, Fusarium graminearum and Alternaria solani) by the mycelium growth inhibition method. The bioassay results indicated that some of the compounds exhibited good antifungal activity against B. cinerea at 100 μg ML−1 compared to other three fungi. In order to better explore the structure–activity relationship (SAR), the EC50 values of target compounds against B. cinerea were measured and assessed. Subsequently, a 3D quantitative structure–activity relationship (3D-QSAR) study was carried out using the comparative molecular field analysis (CoMFA) technique based on the inhibitory activities of tested compounds against B. cinerea. Molecular modelling results revealed fine predictive ability with cross-validated q2 and non-cross-validated r2 values of 0.578 and 0.850, respectively.


 Please wait while we load your content...
Please wait while we load your content...