Tetranuclear lanthanide complexes showing magnetic refrigeration and single molecule magnet behavior†
Abstract
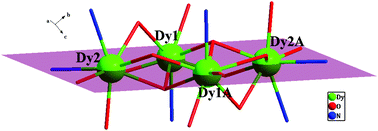
Three tetranuclear lanthanide complexes: {[Ln4(L)6(pbd)4(μ3-OH)2]·2CH3CN} (Ln = Gd (1), Tb (2), Dy (3); HL = 5-(benzylideneamino)quinolin-8-ol, pbd = 1-phenylbutane-1,3-dione) were fabricated and structurally characterized. Compounds 1–3 are isostructural belonging to the triclinic system with space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The core of the complexes contains a tetranuclear arrangement of LnIII ions which is held together by two pyramidal μ3-OH− ions and six μ2 phenol hydroxyl oxygen atoms derived from six L− ligands. Magnetic studies indicated that 1 exhibits cryogenic magnetic refrigeration properties (maximum −ΔSm = 21.41 J K−1 kg−1, ΔH = 7 T at 2.0 K), whereas compound 3 exhibits slow relaxation of the magnetization with an energy barrier (ΔE/kB) of 81.48 K and τ0 = 6.48 × 10−8 s.
. The core of the complexes contains a tetranuclear arrangement of LnIII ions which is held together by two pyramidal μ3-OH− ions and six μ2 phenol hydroxyl oxygen atoms derived from six L− ligands. Magnetic studies indicated that 1 exhibits cryogenic magnetic refrigeration properties (maximum −ΔSm = 21.41 J K−1 kg−1, ΔH = 7 T at 2.0 K), whereas compound 3 exhibits slow relaxation of the magnetization with an energy barrier (ΔE/kB) of 81.48 K and τ0 = 6.48 × 10−8 s.


 Please wait while we load your content...
Please wait while we load your content...