Catalytic performance of graphene quantum dot supported manganese phthalocyanine for efficient oxygen reduction: density functional theory approach
Abstract
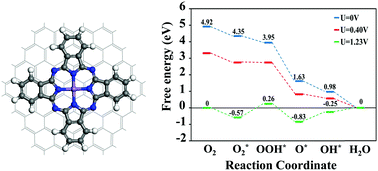
We investigate the catalytic performance of manganese phthalocyanine, as well as manganese phthalocyanine functionalized with a graphene quantum dot matrix, in oxygen-reduction reactions from a thermodynamic perspective. Associative mechanism is found to be energetically favored over the dissociative mechanism for the entire oxygen-reduction reaction process, in both the manganese phthalocyanine and manganese phthalocyanine/graphene quantum dot systems. The initial reduction reaction that forms OOH* is the rate-determining step with reaction barriers of 0.96 eV and 0.83 eV for the manganese phthalocyanine and manganese phthalocyanine/graphene quantum dot systems, respectively. In addition, we perform density of state analyses and construct Gibbs free-energy diagrams for each intermediate step in the overall oxygen-reduction reaction process for both systems, which reveal that the inclusion of the graphene quantum dot increases the number of transferred electrons in the manganese phthalocyanine. Interestingly, the highest operating potential of the manganese phthalocyanine/graphene quantum dot system is higher than that of pristine manganese phthalocyanine. We conclude that the manganese phthalocyanine functionalized with the graphene quantum dot matrix has improved oxygen-reduction reaction activity compared to that of pristine manganese phthalocyanine, and is a potential candidate for use in polymer electrolyte membrane fuel cells.


 Please wait while we load your content...
Please wait while we load your content...