A simple strategy for the detection of Cu(ii), Cd(ii) and Pb(ii) in water by a voltammetric sensor on a TC4A modified electrode
Abstract
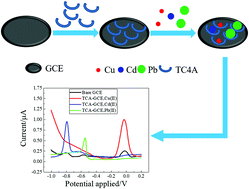
The development of an uncomplicated, fast and low-price method for heavy metal ion detection is essential for environmental detection and pollution control. In this research, an innovative type of electrochemical detection sensor was realized by using a kind of glassy carbon electrode (GCE) modified with thiacalix[4]arene (TC4A). The results indicated that Cu(II), Cd(II) and Pb(II) ions in the sample solution can be detected quickly and simultaneously in the differential pulse anodic stripping voltammetry (DPASV) mode. The test results can reveal whether the concentration of heavy metal ions in wastewater is up to standard. The optimization of multiple experimental parameters including the amount of modification, pH value, deposition time and deposition potential was studied and accomplished. Under the best experimental conditions, the peak value of the voltammetry current increased linearly with the concentration. Within this range, the peak current value and concentration have a very good linear relationship with increasing concentrations of Cu(II), Cd(II) and Pb(II) ions in the ranges of 1.0–2.0 mg L−1, 0.1–1.0 mg L−1 and 1.0–2.0 mg L−1, respectively. And the limits of detection (S/N = 3) are estimated to be 3.0 μg L−1 for Cu(II), 4.0 μg L−1 for Cd(II) and 2.5 μg L−1 for Pb(II), which are lower than those obtained at the unmodified electrodes. Most importantly, the modified electrode was successfully applied in the determination of heavy metals in river water. This innovative electrochemical detection sensor shows high sensitivity, good stability and reproducibility.


 Please wait while we load your content...
Please wait while we load your content...