An expeditious synthetic route to proteomimetic foldamers†
Abstract
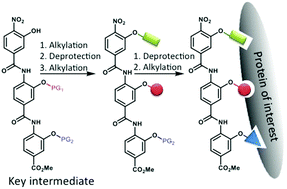
α-Helix proteomimetics such as oligobenzamides have been shown to successfully inhibit a broad array of protein–protein interactions (PPIs) mediated by α-helices. Here we report the synthesis of a protected oligobenzamide intermediate, which can be selectively deprotected and alkylated with desired side chains to rapidly afford a library of α-helix proteomimetics.


 Please wait while we load your content...
Please wait while we load your content...